Alcohol:
Alcohols are organic compounds whose molecules contain hydroxyl group, (-OH) attached to a saturated carbon atom. Alcohols are the hydroxyl deruvatives of hydrocarbons in which one or more hydrogen atoms are teplaced by hydroxyl group.
* Classification of alcohol :
Alcohols are classified as mono, di-, tri,or polyhydric compounds on the basis of one, two, three or more hydroxyl groups present in their molecules as :
Monohydric alcohols are further classified on the basis of hybridisation state of carbon atom to which hydroxyl group is attached. a. Alcohols contauning sp³ C - OH bond:
In these alcohols-OH group is attached to a sp³ hybridised carbon atom of alkyl group. These alcohols are further classified as primary, secondary, and tertiary alcohols in which-OH group is attached to primary, secondary and tertiary carbon atom respectively.
● Allylic alcohols:
In this type os alcohols -OH group is attached to sp³ hybridised carbon atom which is further bonded to a carbon - carbon double bond. Allylic alcohol may be primary, secondary or tertiary.
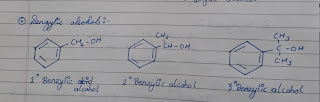
● Benzylic alcohols :
In this type of alcohols -OH group is attached to sp³ hybridised carbon atom which is further bonded to an aromatic ring. Benzylic alcohol may be primary, secondary or tertiary.
b. Alcohols containing sp² C-OH bond :
In this alcohols -OH group is attached to a sp² hybridised carbon atom which is part of a carbon- carbon double bond. These alcohols are known as vinyl alcohols.
*Chemical properties of alcohols:
a. Laboratory tests of alcohols:
1.Litmus test:
Water soluble alcohol can be tested with litmus paper. Aqueous solution of alcohols is neutral to litmus( neither blue nor red litmus change colour).
2. Reaction with base:
Alcohols show no acidic character in aqueous solution, thus, alcohols di not react with either aqueous NaHCO3 or aqueous NaOH. Very weak acidic character of alcohol is revealed in the reaction with active metal. When alcohols are treated with very strong base like alkali metal Na or K they react to give sodium or potassium alkoxide with liberation of hydrogen gas.
3. Distinguish test for alcohols:
Primary, secondary and tertiary alcohols can be distinguish from each other in the laboratory using Lucas reagent.
Alcohols are soluble in Lucas reagent but the product alkyl chloride is not. Hence, the clear solution becomes turbid when product starts forming. Tertiary alcohols react fast and the reagent turns turbid instantaneously. Secondary alcohols turn the reagent turbid slowly. Primary alcohols turn the reagent turbid only on heating.
b. Reactions due to breaking of O-H bond:
1. Acidic character of alcohols:
Ionization of alcohols is represented by the following equilibrium
Electron donating inductive effect(+I effect) of alkyl group destabilizes the alkoxide ion. As a result alcohol does not ionize much in water, and behaves like neutral compound in aqueous medium. Alcohols form ester by reaction with carboxylic acid, acid halids and acid anhydrides. The reaction between alcohol with a carboxylic acid to form an ester is called esterification.
c. Reaction due to breaking of C-O bond in alcohols:
1. Reaction with hydrogen halides:
Alcohols reacts with hydrogen halids to form alkylhalids. Tertiary alcohols react rapidly with hydrogen halids, secondary alcohols react somewhat slower and primary alcohols even more slowly. The order of reactivity of hydrogen halids is
HI>HBr>HCl
2. Reaction with phosphorus halide :
Alcohols react with phosphorus pentahalid(PX5) and phosphorus trihalide(PX3) to form alkyl halids.
3. Dehudration of alcohols to alkenes :
Alcohols when treated with concentrated sulphuric acid or phosphoric acid or alumina undergoes dehydration to form alkene and water. The reaction gives more substituted alkene as the major product, in accordance with Saytzeff rule.
* Physical properties of alcohols:
a. Nature of intermolecular forces:
Alcohols are very polar molecule due to presence of -OH group. The polar -OH groups are held together by the strong intermolecular forces, namely hydrogen bonding.
b.Physical state:
Lower alcohols are colourless, toxic liquids having characteristic alcoholic odour.
c. Boiling points:
The boiling points of alcohols increase with increasd in their molecular mass.
d. Solubility:
Lower alcohols ( having upto three carbons) show appreciable solubility in water due to their ability to form intermolecular hydrogen bonding with water molecule.
1. Methanol is used in industries as a solvent for oils, fats, gum. It is used for drycleaning and also for preparation of perfume. To make ethyl alcohol unfit for drinking.
2. Methyl alcohol is used as a solvent for paints and varnishes.
3. Ehtyl alcohol is used as antifreeze agent in automobile radiators. It is also used as solvent.
*Related topics👇📚❤













Comments
Post a Comment